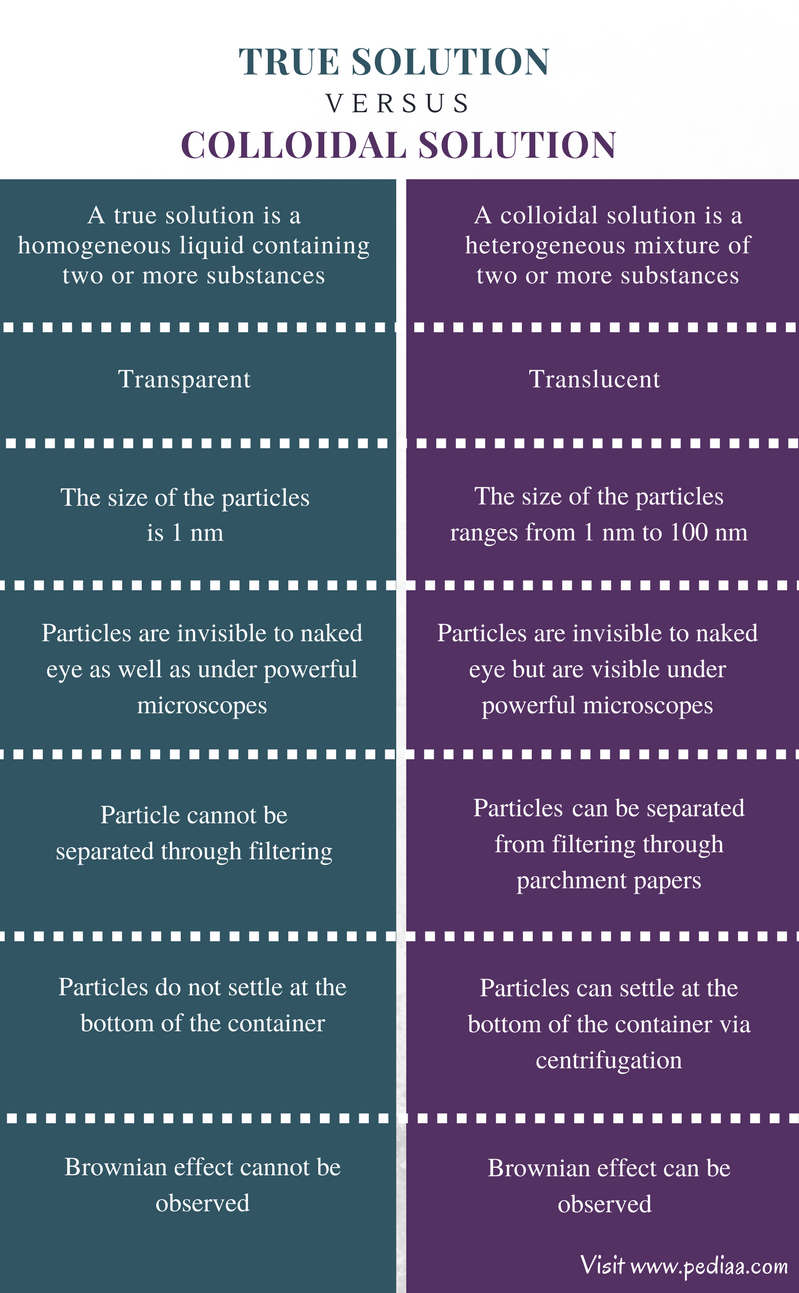
How Is A Colloid Different From A True Solution . colloids can be distinguished from solutions using the tyndall effect. colloidal solution and suspension are heterogeneous mixtures of two or more substances, whereas true solution is a homogeneous mixture. a group of mixtures called colloids (or colloidal dispersions) exhibit properties intermediate between those of. learn what a colloid is in chemistry. Get the definition and colloid examples. colloids are often confused with true homogenous solutions because the individual dispersed particles of a colloid. in this chapter, we will consider the nature of solutions, and examine factors that determine whether a solution will form and. A beam of light passing through a true solution, such as air, is. See how it differs from a solution or suspension. analogous to the identification of solution components as “solute” and “solvent,” the components of a colloid are likewise.
from pediaa.com
See how it differs from a solution or suspension. Get the definition and colloid examples. A beam of light passing through a true solution, such as air, is. colloids are often confused with true homogenous solutions because the individual dispersed particles of a colloid. colloids can be distinguished from solutions using the tyndall effect. a group of mixtures called colloids (or colloidal dispersions) exhibit properties intermediate between those of. learn what a colloid is in chemistry. colloidal solution and suspension are heterogeneous mixtures of two or more substances, whereas true solution is a homogeneous mixture. in this chapter, we will consider the nature of solutions, and examine factors that determine whether a solution will form and. analogous to the identification of solution components as “solute” and “solvent,” the components of a colloid are likewise.
Difference Between True Solution and Colloidal Solution Definition, Properties, Examples
How Is A Colloid Different From A True Solution in this chapter, we will consider the nature of solutions, and examine factors that determine whether a solution will form and. analogous to the identification of solution components as “solute” and “solvent,” the components of a colloid are likewise. A beam of light passing through a true solution, such as air, is. a group of mixtures called colloids (or colloidal dispersions) exhibit properties intermediate between those of. learn what a colloid is in chemistry. in this chapter, we will consider the nature of solutions, and examine factors that determine whether a solution will form and. Get the definition and colloid examples. colloidal solution and suspension are heterogeneous mixtures of two or more substances, whereas true solution is a homogeneous mixture. See how it differs from a solution or suspension. colloids are often confused with true homogenous solutions because the individual dispersed particles of a colloid. colloids can be distinguished from solutions using the tyndall effect.
From www.majordifferences.com
Difference Between True Solutions, Colloidal solution and Suspension Surface Chemistry How Is A Colloid Different From A True Solution learn what a colloid is in chemistry. See how it differs from a solution or suspension. analogous to the identification of solution components as “solute” and “solvent,” the components of a colloid are likewise. colloidal solution and suspension are heterogeneous mixtures of two or more substances, whereas true solution is a homogeneous mixture. in this chapter,. How Is A Colloid Different From A True Solution.
From www.youtube.com
surfaceChemistry True solution, colloidal solution and suspension YouTube How Is A Colloid Different From A True Solution analogous to the identification of solution components as “solute” and “solvent,” the components of a colloid are likewise. in this chapter, we will consider the nature of solutions, and examine factors that determine whether a solution will form and. A beam of light passing through a true solution, such as air, is. See how it differs from a. How Is A Colloid Different From A True Solution.
From exouqcyfe.blob.core.windows.net
What Is Colloidal Suspension In Chemistry at Robert Lemond blog How Is A Colloid Different From A True Solution A beam of light passing through a true solution, such as air, is. learn what a colloid is in chemistry. See how it differs from a solution or suspension. colloids are often confused with true homogenous solutions because the individual dispersed particles of a colloid. colloids can be distinguished from solutions using the tyndall effect. a. How Is A Colloid Different From A True Solution.
From www.youtube.com
Types of mixtures Colloids YouTube How Is A Colloid Different From A True Solution See how it differs from a solution or suspension. colloidal solution and suspension are heterogeneous mixtures of two or more substances, whereas true solution is a homogeneous mixture. colloids are often confused with true homogenous solutions because the individual dispersed particles of a colloid. A beam of light passing through a true solution, such as air, is. . How Is A Colloid Different From A True Solution.
From mungfali.com
Colloid Chart How Is A Colloid Different From A True Solution See how it differs from a solution or suspension. in this chapter, we will consider the nature of solutions, and examine factors that determine whether a solution will form and. Get the definition and colloid examples. colloidal solution and suspension are heterogeneous mixtures of two or more substances, whereas true solution is a homogeneous mixture. A beam of. How Is A Colloid Different From A True Solution.
From www.slideserve.com
PPT IMPURE SUBSTANCES MIXTURES PowerPoint Presentation, free download ID3090813 How Is A Colloid Different From A True Solution See how it differs from a solution or suspension. Get the definition and colloid examples. in this chapter, we will consider the nature of solutions, and examine factors that determine whether a solution will form and. analogous to the identification of solution components as “solute” and “solvent,” the components of a colloid are likewise. colloids are often. How Is A Colloid Different From A True Solution.
From sciencenotes.org
What Is a Colloid? Definition and Examples How Is A Colloid Different From A True Solution Get the definition and colloid examples. in this chapter, we will consider the nature of solutions, and examine factors that determine whether a solution will form and. analogous to the identification of solution components as “solute” and “solvent,” the components of a colloid are likewise. See how it differs from a solution or suspension. a group of. How Is A Colloid Different From A True Solution.
From uen.pressbooks.pub
Colloids Introductory Chemistry How Is A Colloid Different From A True Solution a group of mixtures called colloids (or colloidal dispersions) exhibit properties intermediate between those of. colloids can be distinguished from solutions using the tyndall effect. A beam of light passing through a true solution, such as air, is. colloidal solution and suspension are heterogeneous mixtures of two or more substances, whereas true solution is a homogeneous mixture.. How Is A Colloid Different From A True Solution.
From www.slideserve.com
PPT Matter Properties & Change PowerPoint Presentation, free download ID4473257 How Is A Colloid Different From A True Solution A beam of light passing through a true solution, such as air, is. learn what a colloid is in chemistry. a group of mixtures called colloids (or colloidal dispersions) exhibit properties intermediate between those of. colloidal solution and suspension are heterogeneous mixtures of two or more substances, whereas true solution is a homogeneous mixture. in this. How Is A Colloid Different From A True Solution.
From edurev.in
Difference between true solution, suspension and colloidal sol? EduRev Class 9 Question How Is A Colloid Different From A True Solution Get the definition and colloid examples. analogous to the identification of solution components as “solute” and “solvent,” the components of a colloid are likewise. learn what a colloid is in chemistry. colloidal solution and suspension are heterogeneous mixtures of two or more substances, whereas true solution is a homogeneous mixture. See how it differs from a solution. How Is A Colloid Different From A True Solution.
From pediaa.com
Difference Between True Solution and Colloidal Solution Definition, Properties, Examples How Is A Colloid Different From A True Solution a group of mixtures called colloids (or colloidal dispersions) exhibit properties intermediate between those of. See how it differs from a solution or suspension. analogous to the identification of solution components as “solute” and “solvent,” the components of a colloid are likewise. colloids can be distinguished from solutions using the tyndall effect. in this chapter, we. How Is A Colloid Different From A True Solution.
From www.slideserve.com
PPT Water PowerPoint Presentation, free download ID2182578 How Is A Colloid Different From A True Solution colloids are often confused with true homogenous solutions because the individual dispersed particles of a colloid. colloids can be distinguished from solutions using the tyndall effect. analogous to the identification of solution components as “solute” and “solvent,” the components of a colloid are likewise. See how it differs from a solution or suspension. Get the definition and. How Is A Colloid Different From A True Solution.
From chem.libretexts.org
Colloids Chemistry LibreTexts How Is A Colloid Different From A True Solution colloidal solution and suspension are heterogeneous mixtures of two or more substances, whereas true solution is a homogeneous mixture. analogous to the identification of solution components as “solute” and “solvent,” the components of a colloid are likewise. a group of mixtures called colloids (or colloidal dispersions) exhibit properties intermediate between those of. colloids can be distinguished. How Is A Colloid Different From A True Solution.
From 88guru.com
True solution, Suspension, Colloidal Solutions 88Guru How Is A Colloid Different From A True Solution colloidal solution and suspension are heterogeneous mixtures of two or more substances, whereas true solution is a homogeneous mixture. in this chapter, we will consider the nature of solutions, and examine factors that determine whether a solution will form and. Get the definition and colloid examples. analogous to the identification of solution components as “solute” and “solvent,”. How Is A Colloid Different From A True Solution.
From pediaa.com
Difference Between True Solution and Colloidal Solution Definition, Properties, Examples How Is A Colloid Different From A True Solution See how it differs from a solution or suspension. a group of mixtures called colloids (or colloidal dispersions) exhibit properties intermediate between those of. Get the definition and colloid examples. A beam of light passing through a true solution, such as air, is. in this chapter, we will consider the nature of solutions, and examine factors that determine. How Is A Colloid Different From A True Solution.
From plantlet.org
Mixture Types Solution, Suspension, Colloids & Others Plantlet How Is A Colloid Different From A True Solution colloids are often confused with true homogenous solutions because the individual dispersed particles of a colloid. a group of mixtures called colloids (or colloidal dispersions) exhibit properties intermediate between those of. analogous to the identification of solution components as “solute” and “solvent,” the components of a colloid are likewise. colloids can be distinguished from solutions using. How Is A Colloid Different From A True Solution.
From quizizz.com
Describe the appearance of Colloids and Suspension. Quizizz How Is A Colloid Different From A True Solution learn what a colloid is in chemistry. colloids can be distinguished from solutions using the tyndall effect. Get the definition and colloid examples. See how it differs from a solution or suspension. A beam of light passing through a true solution, such as air, is. analogous to the identification of solution components as “solute” and “solvent,” the. How Is A Colloid Different From A True Solution.
From www.myshared.ru
Презентация на тему "Colloidal systems. Classes of solution True solutions Colloids Suspensions How Is A Colloid Different From A True Solution learn what a colloid is in chemistry. colloids are often confused with true homogenous solutions because the individual dispersed particles of a colloid. Get the definition and colloid examples. colloidal solution and suspension are heterogeneous mixtures of two or more substances, whereas true solution is a homogeneous mixture. analogous to the identification of solution components as. How Is A Colloid Different From A True Solution.